Fatty acid catabolism includes the complete oxidation (β oxidation) of fatty acid to yield acetyl CoA which has different fates. Fatty acids are hydrocarbons with energy of complete oxidation (about 38 kJ/g) more than twice that for the same weight of carbohydrate or protein. To overcome the stability of C-C bonds in a fatty acid, the carboxyl group at C-1 is activated by attachment to coenzyme A, which allows stepwise oxidation of the fatty acyl group at the C-3, or β position – hence the name β oxidation.
The complete oxidation of fatty acid to CO2 and H2O takes place in 3 stages:
1) Oxidation of long-chain fatty acids to 2 carbon fragments, acetyl-CoA (β oxidation)
2) Oxidation of acetyl-CoA to CO2 in the CAC (and to other products in special conditions).
3) The transfer of electrons from reduced electron carriers to the mitochondrial respiratory chain.
It includes oxidation of both saturated even chain fatty acid and unsaturated fatty acid and fatty acid with odd number of carbons. It also includes ω and α oxidation.
Fatty acid activation and transportation to mitochondria
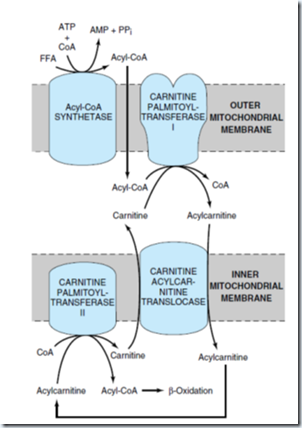
The enzymes of fatty acid oxidation lie in mitochondrial matrix. Fatty acid with chain length 12 or fewer do not require membrane transporter but FA with more than this which constitute the majority obtained in the diet or released from adipose tissue should undergo 3 enzymatic reactions of carnitine shuttle to enter mitochondrial matrix.
1) Acyl-CoA synthetase (+nt in outer mitochondrial membrane) catalyzed reaction.
The carboxylate ion is adenylylated by ATP, to form a fatty acyl-adenylate and PPi. The PPi is immediately hydrolyzed to two molecules of Pi.
The thiol group of coenzyme A attacks the acyl-adenylate (a mixed anhydride), displacing AMP and forming the thioester fatty acyl-CoA.
The direct condensation of fatty acid with coenzyme A is endergonic, but the formation of fatty acyl-CoA is made exergonic by stepwise removal of two phosphoryl groups from ATP. The sum of reaction 1 and 2 reaction is energetically equivalent to exergonic hydrolysis of ATP to AMP and PPi (∆G’0 = -45 kJ/mol) and the endergonic formation of fatty acyl-CoA (∆G’0 = 31.4 kJ/mol). Formation of fatty acyl-CoA is made energetically favorable by PPi hydrolysis by inorganic pyrophosphatase (-19.2 kJ/mol)
ATP +2H2O AMP + 2Pi ∆G’0 = -64.8 kJ/mol {-45.6 + (-19.2) kJ/mol}
The overall reaction of this process is.

Produced fatty acyl-CoA can be transported to mitochondria and oxidized to form ATP or utilized in the cytosol to form membrane lipids.
2) Carnitine acyltransferase I catalyzed reaction:
Fatty acids to be transported to mitochondria are attached to the –OH group of carnitine to form fatty acyl-carnitine, this transesterification is catalyzed by this enzyme in the outer membrane.

Formed from lysine and methionine in liver and kidney

The fatty acyl-carnitine ester enters the matrix by facilitated diffusion through the acyl carnitine/carnitine transporter of the inner mitochondrial membrane.
3) Carnitine acyl-transferase II (carnitine palmitoyltransferase-II) catalyzed reaction:
Fatty acyl group is enzymatically transferred from carnitine to intramitochondrial coenzyme A catalyzed by this enzyme. This enzyme is located in the inner face of inner mitochondrial membrane.
This three pathway links the coenzyme of cytosol and in mitochondria. Coenzyme A in the mitochondrial matrix is used in oxidative degradation of pyruvate, fatty acids, and some amino acids, whereas cytosolic coenzyme A is used in the biosynthesis of fatty acids.
This carnitine-mediated entry process is the rate limiting step for oxidation of fatty acid.
Oxidation of Fatty acid
Mitochondrial fatty acid oxidation takes place in 3 stages.
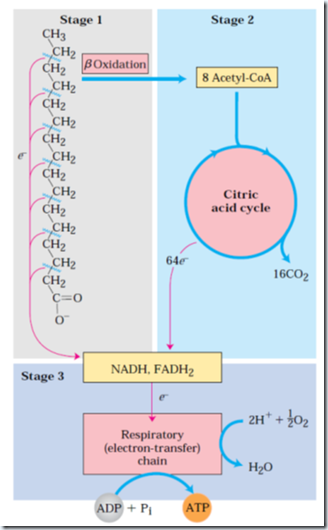
Stage 1: β oxidation- There is successive removal of fatty acids’ two carbon units in the form of acetyl-CoA, starting from the carboxyl end of the fatty acyl chain. At the end of cycle last two carbons remain as acetyl-CoA. (E.g. 16-C palmitic acid undergoes 7 cycles to give eight two carbon acetyl groups of acetyl-CoA). Formation of each acetyl-CoA requires removal of 4 hydrogen atoms (2 pair of electrons and four H+) from the fatty acyl moiety by dehydrogenase.
Stage 2: The acetyl group of acetyl-CoA is oxidized to CO2 in the CAC, which also takes place in mitochondrial matrix.
Stage 3: NADH produced in the first stage and FADH2 in the second stage, which now donates electrons to the mitochondrial respiratory chain and ATP formation.
The β oxidation of saturated fatty acid
This has 4 basic steps.
1st step:
Here in each pass through this 4 step, one acetyl residue is removed in the form of acetyl-CoA from the carboxyl end of the fatty acyl chain.
Acyl-CoA dehydrogenase is specific for a range of fatty acyl chain length: Very-long-chain acyl-CoA dehydrogenase (VLCAD), acting on fatty acids of 12 to 18 carbons; medium-chain acyl-CoA dehydrogenase (MCAD), acting on fatty acid of 4 to 14 carbons; and short-chain (SCAD), acting on fatty acids of 4 to 8 carbons. All three isozymes are flavoproteins with FAD as a prosthetic group. It is present in inner mitochondrial membrane.
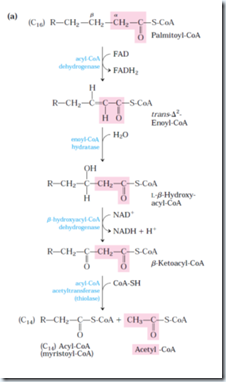


The electrons removed from fatty acyl-CoA are transferred to FAD, and it donates a pair of electron to electron carrier of the mitochondrial respiratory chain, the electron-transferring Flavoprotein (ETF) with the concomitant synthesis of about 1.5 ATP molecules per electron pair. This action is similar to Succinate dehydrogenase.
2nd step: Action of enoyl-CoA hydratase is analogous to the fumarase reaction of CAC, in which H2O adds across a α-β double bond.
3rd step: This enzyme is specific for L-stereoisomer. The NADH formed donates a electron pair to NADH dehydrogenase, an electron carrier of the respiratory chain and 2.5 molecules of ATP is formed per pair. This enzyme catalysis is analogous to malate dehydrogenase of CAC.
4th step: The reaction of this step is called thiolysis analogous to hydrolysis. The ketone function on the β carbon (C-3) makes it a good target for nucleophilic attack by the –SH group of coenzyme A.
For fatty acyl chains of 12 or more carbons, the reactions are catalyzed by a multienzyme complex associated with inner mitochondrial membrane, the trifunctional protein (TEF). This is a heterooctamer of α4β4 subunits. Each α subunits has two active sites, the enoyl-CoA hydratase and the β-hydorxyacyl-CoA dehydrogenase; the β-subunit contains the thiolase activity. This provides good substrate channeling without escaping. When TEF has shorten the fatty acyl chain to 12 or fewer carbons, further oxidations are catalyzed by a set of four soluble enzymes in the matrix.
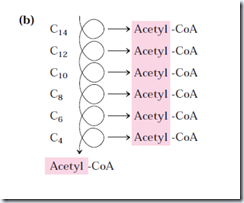
Here, six more pass through the pathway yield seven more molecules of acetyl-CoA, the seventh arising from the last two carbon atoms of the 16 carbon chain. Eight molecules of acetyl-CoA are formed in all.
The overall equation of palmitoyl-CoA oxidation is given as follows;

During this β oxidation process of one fatty acyl CoA, four molecule of ATP are formed for each two carbon unit removed in one pass through the sequence, through NADH and FADH2 mediated electron transfer.
Transfer of electrons from NADH or FADH2 to O2 yields one H2O per electron pair. Reduction of O2 by NADH also consumes one H+ per NADH molecule.
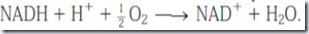
Thus in hibernating animals, fatty acid oxidation provides metabolic energy, heat, and water.
Acetyl CoA can be further oxidized in the Citric Acid Cycle.
The oxidation of acetyl-CoA formed form palmitoyl CoA, in CAC is given by:

The overall oxidation of palmitoyl CoA including oxidation of acetyl CoA in CAC along with respiratory chain, is given by,


Since 2 ATP are utilized in the activation of palmitate to palmitoyl CoA, the net energy yield is 106 ATP.
Transport in the respiratory chain of electrons from FADH2 and NADH will lead to the synthesis of five high-energy phosphates (Chapter 12) for each of the first seven acetyl-CoA molecules formed by β-oxidation of palmitate (7 × 5 = 35). A total of 8 mol of acetyl-CoA is formed, and each will give rise to 12 mol of ATP on oxidation in the citric acid cycle, making 8 × 12 = 96 mol. Two must be subtracted for the initial activation of the fatty acid, yielding a net gain of 129 mol of ATP per mole of palmitate, or 129 × 51.6* = 6656 kJ. This represents 68% of the free energy of combustion of palmitic acid.
Oxidation of unsaturated fatty acid requires two additional reactions
Most of the fatty acids in Tg and phospholipids of animals and plants are unsaturated. The double bonds are in cis configuration and cannot be acted by enoyl-CoA hydratase. So, two additional enzymes are required for β oxidation.
For, e.g. Oleate (an abundant 18 Carbon monounsaturated fatty acid with a cis double bond (∆9). All other steps are same except on additional enzyme.

Thus 9 acetyl-CoA are formed from 18 C oleic acid.
E.g. 18-C linoleate (18-C polyunsaturated fatty acid having cis-∆9, cis-∆12 configuration).

NADPH is supplied by intramitochondrial sources such as glutamate dehydrogenase, isocitrate dehydrogenase and NAD(P)H transdehydrogenase.
Thus, the overall conversion of linoleate to nine molecules of acetyl-CoA.
Complete oxidation of odd number of fatty acids requires three extra reactions
Most naturally occurring lipids contain even numbered fatty acid, but odd numbers are common in many plants and some marine organisms. E.g. propionate (odd number fatty acid) of animal origin can enter human diet.
The oxidation is same as in even numbered fatty acid but the substrate for the last pass through the β oxidation sequence is a fatty acyl-CoA with a five carbon fatty acid, thus the products are acetyl-CoA and propionyl-CoA. Acetyl-CoA is oxidized via CAC but propionyl-CoA enters different pathway involving 3 enzymes. Propionate may be used in adipose tissue and mammary gland as priming molecule for synthesis of odd number fatty acids.

Vitamin B12 deficiency results in excretion of large amounts of methylmalonate (methylmalonic aciduria)
In the first step, biotin is a cofactor and is similar to pyruvate Carboxylase reaction. CO2 or HCO3-) is activated by attachment to biotin before its transfer to the substrate (propionate moiety). Energy required for the formation of carboxybiotin intermediate is provided by ATP.
In the third step the enzyme require coenzyme 5’-deoxyadenosylcobalamin, or coenzyme B12. It is a cofactor for this kind of mutase reaction.


1) The Co-C bond undergoes hemolytic cleavage, yielding Co2+ and the 5’-deoxyadenosyl free radical.
2) The radical is converted to 5’-deoxyadenosine by abstraction of a hydrogen atom from the substrate, producing a substrate radical.
3) The substrate radical is rearranged, producing a new radical with the carbon skeleton of the product. For methyl-malonyl-CoA mutase, the migrating group (X) is –CO-SCoA.
4) Hydrogen from the 5’ –CH3 of the deoxyadenosine is returned to the product like radical, forming product.
5) The bond between the 5’ – CH2 of the deoxyadenosyl radical and cobalt is re-formed, regenerating the B12 cofactor in its Co3+ form, ready to undergo another reaction.
· The migrating hydrogen in step 2 and 4 never exists as a free species and does not exchange with the hydrogen of surrounding water molecules.
The complete oxidation of fatty acid to CO2 and H2O takes place in 3 stages:
1) Oxidation of long-chain fatty acids to 2 carbon fragments, acetyl-CoA (β oxidation)
2) Oxidation of acetyl-CoA to CO2 in the CAC (and to other products in special conditions).
3) The transfer of electrons from reduced electron carriers to the mitochondrial respiratory chain.
It includes oxidation of both saturated even chain fatty acid and unsaturated fatty acid and fatty acid with odd number of carbons. It also includes ω and α oxidation.
Fatty acid activation and transportation to mitochondria
The enzymes of fatty acid oxidation lie in mitochondrial matrix. Fatty acid with chain length 12 or fewer do not require membrane transporter but FA with more than this which constitute the majority obtained in the diet or released from adipose tissue should undergo 3 enzymatic reactions of carnitine shuttle to enter mitochondrial matrix.
1) Acyl-CoA synthetase (+nt in outer mitochondrial membrane) catalyzed reaction.
The carboxylate ion is adenylylated by ATP, to form a fatty acyl-adenylate and PPi. The PPi is immediately hydrolyzed to two molecules of Pi.
The thiol group of coenzyme A attacks the acyl-adenylate (a mixed anhydride), displacing AMP and forming the thioester fatty acyl-CoA.

The direct condensation of fatty acid with coenzyme A is endergonic, but the formation of fatty acyl-CoA is made exergonic by stepwise removal of two phosphoryl groups from ATP. The sum of reaction 1 and 2 reaction is energetically equivalent to exergonic hydrolysis of ATP to AMP and PPi (∆G’0 = -45 kJ/mol) and the endergonic formation of fatty acyl-CoA (∆G’0 = 31.4 kJ/mol). Formation of fatty acyl-CoA is made energetically favorable by PPi hydrolysis by inorganic pyrophosphatase (-19.2 kJ/mol)
ATP +2H2O AMP + 2Pi ∆G’0 = -64.8 kJ/mol {-45.6 + (-19.2) kJ/mol}
The overall reaction of this process is.

Produced fatty acyl-CoA can be transported to mitochondria and oxidized to form ATP or utilized in the cytosol to form membrane lipids.
2) Carnitine acyltransferase I catalyzed reaction:
Fatty acids to be transported to mitochondria are attached to the –OH group of carnitine to form fatty acyl-carnitine, this transesterification is catalyzed by this enzyme in the outer membrane.

Formed from lysine and methionine in liver and kidney

The fatty acyl-carnitine ester enters the matrix by facilitated diffusion through the acyl carnitine/carnitine transporter of the inner mitochondrial membrane.
3) Carnitine acyl-transferase II (carnitine palmitoyltransferase-II) catalyzed reaction:
Fatty acyl group is enzymatically transferred from carnitine to intramitochondrial coenzyme A catalyzed by this enzyme. This enzyme is located in the inner face of inner mitochondrial membrane.
This three pathway links the coenzyme of cytosol and in mitochondria. Coenzyme A in the mitochondrial matrix is used in oxidative degradation of pyruvate, fatty acids, and some amino acids, whereas cytosolic coenzyme A is used in the biosynthesis of fatty acids.
This carnitine-mediated entry process is the rate limiting step for oxidation of fatty acid.
Oxidation of Fatty acid
Mitochondrial fatty acid oxidation takes place in 3 stages.

Stage 1: β oxidation- There is successive removal of fatty acids’ two carbon units in the form of acetyl-CoA, starting from the carboxyl end of the fatty acyl chain. At the end of cycle last two carbons remain as acetyl-CoA. (E.g. 16-C palmitic acid undergoes 7 cycles to give eight two carbon acetyl groups of acetyl-CoA). Formation of each acetyl-CoA requires removal of 4 hydrogen atoms (2 pair of electrons and four H+) from the fatty acyl moiety by dehydrogenase.
Stage 2: The acetyl group of acetyl-CoA is oxidized to CO2 in the CAC, which also takes place in mitochondrial matrix.
Stage 3: NADH produced in the first stage and FADH2 in the second stage, which now donates electrons to the mitochondrial respiratory chain and ATP formation.
The β oxidation of saturated fatty acid
This has 4 basic steps.
1st step:
Here in each pass through this 4 step, one acetyl residue is removed in the form of acetyl-CoA from the carboxyl end of the fatty acyl chain.
Acyl-CoA dehydrogenase is specific for a range of fatty acyl chain length: Very-long-chain acyl-CoA dehydrogenase (VLCAD), acting on fatty acids of 12 to 18 carbons; medium-chain acyl-CoA dehydrogenase (MCAD), acting on fatty acid of 4 to 14 carbons; and short-chain (SCAD), acting on fatty acids of 4 to 8 carbons. All three isozymes are flavoproteins with FAD as a prosthetic group. It is present in inner mitochondrial membrane.



The electrons removed from fatty acyl-CoA are transferred to FAD, and it donates a pair of electron to electron carrier of the mitochondrial respiratory chain, the electron-transferring Flavoprotein (ETF) with the concomitant synthesis of about 1.5 ATP molecules per electron pair. This action is similar to Succinate dehydrogenase.
2nd step: Action of enoyl-CoA hydratase is analogous to the fumarase reaction of CAC, in which H2O adds across a α-β double bond.
3rd step: This enzyme is specific for L-stereoisomer. The NADH formed donates a electron pair to NADH dehydrogenase, an electron carrier of the respiratory chain and 2.5 molecules of ATP is formed per pair. This enzyme catalysis is analogous to malate dehydrogenase of CAC.
4th step: The reaction of this step is called thiolysis analogous to hydrolysis. The ketone function on the β carbon (C-3) makes it a good target for nucleophilic attack by the –SH group of coenzyme A.
For fatty acyl chains of 12 or more carbons, the reactions are catalyzed by a multienzyme complex associated with inner mitochondrial membrane, the trifunctional protein (TEF). This is a heterooctamer of α4β4 subunits. Each α subunits has two active sites, the enoyl-CoA hydratase and the β-hydorxyacyl-CoA dehydrogenase; the β-subunit contains the thiolase activity. This provides good substrate channeling without escaping. When TEF has shorten the fatty acyl chain to 12 or fewer carbons, further oxidations are catalyzed by a set of four soluble enzymes in the matrix.

Here, six more pass through the pathway yield seven more molecules of acetyl-CoA, the seventh arising from the last two carbon atoms of the 16 carbon chain. Eight molecules of acetyl-CoA are formed in all.
The overall equation of palmitoyl-CoA oxidation is given as follows;
During this β oxidation process of one fatty acyl CoA, four molecule of ATP are formed for each two carbon unit removed in one pass through the sequence, through NADH and FADH2 mediated electron transfer.
Transfer of electrons from NADH or FADH2 to O2 yields one H2O per electron pair. Reduction of O2 by NADH also consumes one H+ per NADH molecule.
Thus in hibernating animals, fatty acid oxidation provides metabolic energy, heat, and water.
Acetyl CoA can be further oxidized in the Citric Acid Cycle.
The oxidation of acetyl-CoA formed form palmitoyl CoA, in CAC is given by:

The overall oxidation of palmitoyl CoA including oxidation of acetyl CoA in CAC along with respiratory chain, is given by,


Since 2 ATP are utilized in the activation of palmitate to palmitoyl CoA, the net energy yield is 106 ATP.
Transport in the respiratory chain of electrons from FADH2 and NADH will lead to the synthesis of five high-energy phosphates (Chapter 12) for each of the first seven acetyl-CoA molecules formed by β-oxidation of palmitate (7 × 5 = 35). A total of 8 mol of acetyl-CoA is formed, and each will give rise to 12 mol of ATP on oxidation in the citric acid cycle, making 8 × 12 = 96 mol. Two must be subtracted for the initial activation of the fatty acid, yielding a net gain of 129 mol of ATP per mole of palmitate, or 129 × 51.6* = 6656 kJ. This represents 68% of the free energy of combustion of palmitic acid.
Oxidation of unsaturated fatty acid requires two additional reactions
Most of the fatty acids in Tg and phospholipids of animals and plants are unsaturated. The double bonds are in cis configuration and cannot be acted by enoyl-CoA hydratase. So, two additional enzymes are required for β oxidation.
For, e.g. Oleate (an abundant 18 Carbon monounsaturated fatty acid with a cis double bond (∆9). All other steps are same except on additional enzyme.

Thus 9 acetyl-CoA are formed from 18 C oleic acid.
E.g. 18-C linoleate (18-C polyunsaturated fatty acid having cis-∆9, cis-∆12 configuration).

NADPH is supplied by intramitochondrial sources such as glutamate dehydrogenase, isocitrate dehydrogenase and NAD(P)H transdehydrogenase.
Thus, the overall conversion of linoleate to nine molecules of acetyl-CoA.
Complete oxidation of odd number of fatty acids requires three extra reactions
Most naturally occurring lipids contain even numbered fatty acid, but odd numbers are common in many plants and some marine organisms. E.g. propionate (odd number fatty acid) of animal origin can enter human diet.
The oxidation is same as in even numbered fatty acid but the substrate for the last pass through the β oxidation sequence is a fatty acyl-CoA with a five carbon fatty acid, thus the products are acetyl-CoA and propionyl-CoA. Acetyl-CoA is oxidized via CAC but propionyl-CoA enters different pathway involving 3 enzymes. Propionate may be used in adipose tissue and mammary gland as priming molecule for synthesis of odd number fatty acids.

Vitamin B12 deficiency results in excretion of large amounts of methylmalonate (methylmalonic aciduria)
In the first step, biotin is a cofactor and is similar to pyruvate Carboxylase reaction. CO2 or HCO3-) is activated by attachment to biotin before its transfer to the substrate (propionate moiety). Energy required for the formation of carboxybiotin intermediate is provided by ATP.
In the third step the enzyme require coenzyme 5’-deoxyadenosylcobalamin, or coenzyme B12. It is a cofactor for this kind of mutase reaction.


1) The Co-C bond undergoes hemolytic cleavage, yielding Co2+ and the 5’-deoxyadenosyl free radical.
2) The radical is converted to 5’-deoxyadenosine by abstraction of a hydrogen atom from the substrate, producing a substrate radical.
3) The substrate radical is rearranged, producing a new radical with the carbon skeleton of the product. For methyl-malonyl-CoA mutase, the migrating group (X) is –CO-SCoA.
4) Hydrogen from the 5’ –CH3 of the deoxyadenosine is returned to the product like radical, forming product.
5) The bond between the 5’ – CH2 of the deoxyadenosyl radical and cobalt is re-formed, regenerating the B12 cofactor in its Co3+ form, ready to undergo another reaction.
· The migrating hydrogen in step 2 and 4 never exists as a free species and does not exchange with the hydrogen of surrounding water molecules.


No comments:
Post a Comment