About 99% of calcium occurs in
bone, along with calcium 85% of phosphate and 55% magnesium. The concentration
of these minerals depends on net effect of bone mineralization, intestinal
absorption and renal excretion. PTH and 1, 25-dihydroxyvitamin D are the
principle hormones regulating these three processes.
Calcium is the 5th most abundant element, and most prevalent cation. Average human body contains approximately 1 kg of calcium. It is found in skeleton (99%) as hydroxyapatite crystal, soft tissues (1%), and extracellular fluid (<0.2%).
Hypocalcemia
Initial lab assessment is
measurement of renal function and measurement of serum albumin and magnesium
concentrations. Other parameters are low vitamin D, PTH or sometimes high PTH
due to resistance, and high serum ALP. Large amounts of burn cream contain
polyethylene glycols which are absorbed and metabolized to dicarboxylic acid
that bind calcium. Patient develops elevated total calcium but low free
calcium, along with metabolic acidosis and increased serum osmolality from
glycols.
Hypercalcemia
Photometric methods
i.
O-Cresolphthalein complexon method
ii.
Arsenazo III method
iii.
Clark and collip method
Atomic Absorption Spectrometry(AAS) method
Specimen requirement
Specimen requirement for ISE
Calcium is the 5th most abundant element, and most prevalent cation. Average human body contains approximately 1 kg of calcium. It is found in skeleton (99%) as hydroxyapatite crystal, soft tissues (1%), and extracellular fluid (<0.2%).
In blood virtually all of the
calcium is in plasma, with normal concentration of 9.5 mg/dl (2.38 mmol/L). 50%
is free (ionized) which is active form, 40% is protein bound mainly albumin
followed by globulin and 10% is complexed with small anions like lactate,
phosphate, bicarbonate and citrate. The concentration of free form is regulated
by calcium regulating hormones PTH and 1, 25-dihydroxyvitamin D. It binds to
negatively charged proteins so binding is pH dependent more binding occurring
in alkaline pH. In multiple myeloma high concentration of globulin may bind
calcium reducing free pool.
The skeleton is a major reservoir
for providing calcium for both extracellular and intracellular pools.
Intracellularly calcium is involved muscle contraction, release of granules in
hormone secretion, cell division, etc. Extracellular calcium provides calcium
for maintenance of intracellular calcium, bone mineralization, blood
coagulation, and plasma membrane potential.
Calcium is absorbed from GI by two
mechanisms one is active transport involving vitamin D, calcitriol in duodenum
and jejunum and another is by passive method in colon. The absorption of
calcium is influenced by dietary constituents. The presence of anions such as
phosphate, oxalate (in green vegetables), and phytate (in cereals) diminishes
calcium solubility and thus absorption.
CLINICAL SIGNIFICANCE
Hypocalcemia
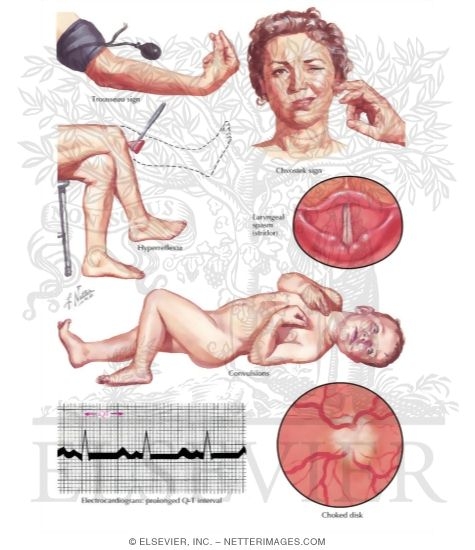 |
| Fig. Hypocalcemia signs |
This may be due to reduced albumin
bound calcium, free calcium or both. Hypoalbuminemia is the cause of
pseudohypocalcemia. Other conditions resulting to hypocalcemia are chronic
liver disease, nephrotic syndrome, congestive heart failure, and malnutrition,
osteomalacia and rickets, etc. but common causes are chronic renal failure and
hypomagnesemia. In chronic renal failure, hypoproteinemia, hyperphosphatemia,
low serum vitamin D (reduced synthesis because of inadequate renal mass) and/or
skeletal resistance to PTH contribute to hypocalcemia. Magnesium deficiency
impairs PTH secretion and cause PTH end-organ resistance. Inherited resistance
of PTH leads to pseudohypoparathyroidism and thus hypocalcemia e.g. in
pseudohypoparathyroidism type I (Albright’s hereditary osetodystrophy) is due
to reduction in guanine nucleotide regulatory complex in adenylate cyclase
complex. Vitamin D deficiency is also associated with hypocalcemia and is due
to impaired intestinal absorption of calcium and skeletal resistance to PTH.
Clinically hypocalcemia presents with neuromuscular hyperexcitability, such as
tetany, paresthesia, and seizures.
Hypercalcemia
This occurs due to excessive bone
resorption like in malignancy. Failure of kidney to excrete filtered calcium
also caused hypercalcemia. It may be caused by increased intestinal absorption
(vitamin D intoxication), increased renal retention (thiazide diuretics),
increased skeletal resorption, or combination of mechanisms (primary
hyperparathyroidism which occur due to adenoma, hyperplasia, etc.) is the most
common cause of hypercalcemia (90%) in outpatients and malignancy (95%) in
inpatients. Primary hyperPTH is characterized by excessive secretion of PTH
causing hypercalcemia. The most common symptoms are fatigue, malaise, weakness
and mild hypercalcemia, etc. Increase in albumin or globulins as in multiple
myeloma binds more calcium and cause to increase total calcium. Hypercalcemia
causes hypercalciuria which can lead to renal calculi. In sarcoidosis and other
granulomatous disease tissue contains 25-hydroxyvitamin D-1α-hydroxylase
required to produce active vitamin D.
 |
| Fig. Causes of Hypercalcemia |
Laboratory analysis includes
measurement of serum calcium (ideally free calcium), albumin, PTH, 1, 25
vitamin D.
Hypercalcemia affects from 0.1 to 1% of the population. The
widespread ability to measure blood calcium since the 1960s has improved
detection of the condition, and today most patients with hypercalcemia have no
symptoms. Women over the age of 50 are most likely to be hypercalcemic, usually
due to primary hyperparathyroidism.
MEASUREMENT OF CALCIUM
Measurement of calcium includes
either free or total calcium. The term ionized calcium is misnomer because all
plasma calcium is ionized either free or bound form so, free form is
appropriate. Free calcium is the best indicator of calcium status as it is the
active form and tightly regulated by PTH and vitamin D. ISE and other
autoanalyzers are available to measure free but preferably total calcium. Corrected calcium is often used that corrects
measured calcium with albumin.
Corrected total calcium (mg/dL) =
total calcium (mg/dl) + 0.84 (4-albumin [g/dl])
; 4 represents the average albumin level in
g/dL.
In other words, each 1 g/dL decrease of albumin will decrease
0.8 mg/dL in measured serum Ca and thus 0.8 must be added to the measured
Calcium to get a corrected Calcium value.
Factors affecting protein binding
to calcium includes, altered albumin or globulins, heparin, pH, free fatty
acids, bilirubin, etc. Whereas factors altering complex formation includes,
citrate, bicarbonate, lactate, pyruvate, sulfate, anion gap, etc.
The methods of total calcium
measurements includes
Photometric methods
i.
O-Cresolphthalein complexon method
In alkaline solution, the
metal-complexing dye CPC forms a red chromophore with calcium measured at 580
nm. The sample is diluted with acid to release protein-bound and complexed
calcium. Use of Hydroxyquinolone, pH 12 and measuring at 580 nm are used to
prevent interference from magnesium. Diethylamine is used to produce alkaline
pH.
ii.
Arsenazo III method
Arsenazo III at mild acidic
condition (pH 6) has higher affinity to calcium than magnesium. Imidazole is
used to buffer the reaction. Interference from most biological pigments is
reduced by measuring the complex at 650 nm.
iii.
Clark and collip method
Serum total calcium is precipitated
as calcium oxalate, which is washed with ammonia and dissolved in acid. Oxalic
acid thus formed is titrated at 700C- 800C against
standard permanganate. From the titre value the calcium content of serum is
calculated.
Calcium + ammonium oxalate -------> calcium
oxalate (ppt)
2KMNO4 + 3H2SO4
+ 5(COOH)2 -----> K2SO4
+ 2MnSO4 + 8H2O + 10CO2
Atomic Absorption Spectrometry(AAS) method
Use of AAS is the reference method
for measuring total serum calcium and IDMS is the definitive method. In this
method, the specimen is first diluted with lanthanum-HCL, and then aspirated
into an air-acetylene flame, where the ground state calcium atoms absorb
incident light from a calcium hollow cathode lamp (422.7 nm). The amount of
light absorbed is measured by phototube or detector after the 422.7 nm
resonance line is isolated with the monochromator. Absorbance is directly
proportional to the number of ground state calcium atoms in the flame.
Dilution with lanthanum-HCl reduces
interference from protein, phosphate, citrate, sulfate and other anions. Phosphate
causes the greatest interference because calcium phosphate complexes are not
dissociated readily by air-acetylene flame. Lanthanum-HCl dissociates complexes
ensuring that all fractions of calcium are measured. Dilution reduces viscosity
which improves aspiration rate.
Specimen requirement
Serum and heparinized plasma are
the preferred specimens. Hemolysis, icterus, lipemia, paraproteins, Hb,
bilirubin and Mg interfere with the test.
Measurement of free (Ionized) calcium
These used ISE that determine free
calcium from whole blood. The instrument contains calcium ion-selective,
reference, and pH electrodes. Sensitive potentiometers measure the voltage
difference between the calcium or pH and reference electrodes for calibrating
solutions or samples. Calcium ISE contains a calcium selective membrane, which
encloses an inner reference solution of calcium chloride often containing
saturated AgCl and physiological concentration of NaCl and KCl and an internal
reference electrode. The reference electrode usually of Ag/AgCl is immersed in
this inner reference solution. The electrochemical systems include reference
electrodes, calcium sensitive electrode and salt bridge like in pH electrode.
The potential difference across the cell is logarithmically related to the
activity of free calcium ions in sample by Nernst’s equation.
Specimen requirement for ISE
Heparinized whole blood,
heparinized plasma or serum can be used. CO2 loss should be
prevented otherwise this will increase in pH as occurs when specimen are
exposed to air. Ideally whole blood specimen should be analyzed within 15 to 30
minutes of sampling. But 1 hr at room temperature and 4 hr at 40C is
reported to be stable. Serum specimens shows greater stability. The practice of
using aerobic specimens for measurement of free calcium should be abandoned.
PATIENT PREPARATION FOR CALCIUM MEASUREMENT
Due to tourniquet application total
calcium is altered but not free calcium and this is due to venous occlusion and
there is efflux of water from the vascular compartment during stasis. Fist
clenching or other forearm exercise should be avoided before phlebotomy,
because forearm exercise causes decrease in pH (lactic acid production) and an
increase in free calcium. Standing decreases intravascular water and increase
the total calcium. Prolonged immobilization and bed rest can decrease bone
density and increase total and free calcium.
REFERENCE INTERVALS
Total calcium = 8.6-10.2 mg/dL (2.15 – 2.55 mmol/L)
Free calcium = 4.6 -5.3 mg/dL (1.15
– 1.33 mmol/L)
Since free calcium is affected by pH, it is recommended that pH
be measured and reported with all free calcium determinations.
Free calcium is more useful than
total calcium determination in hospital patients, especially those undergoing
major surgery who have received citrated blood or platelets, heparin,
bicarbonate. Rapid measurement of free calcium, blood gases, and potassium
permits maintenance of good cardiac function during surgery. Free calcium is
more useful than total in diagnosis of hypercalcemia as in primary
hyperparathyroidism where there is increase in free calcium than total and also
malignancy.
Measurement of calcium in urine
reflects intestinal absorption, skeletal resorption and renal tubular
filtration and reabsorption. The measurement is useful in assessing renal stone
disease and high-turnover osteoporosis. Calcium oxalate crystals can be seen in
case of stones. Acidification of urine is done by 6 mol/L HCl to prevent
calcium salt precipitation.



No comments:
Post a Comment