An adult contains 600 g of phosphorus in inorganic and
organic phosphates, of which about 85% is in skeleton and rest in soft tissue
and extracellular fluid. Though plasma has both organic and inorganic phosphate
but inorganic phosphate (H2PO4- and HPO42-) is
measured. Approximately 10% is protein bound and 35% complexed with sodium,
calcium, magnesium; and remainder 55% is free. The organic phosphates are
located within the cell of blood.
Inorganic phosphate is a major component of hydroxyapatite
in bone and is the source of intracellular and extracellular pool. Organic
phosphate in cells is found to be incorporated into nucleic acid,
phospholipids, phosphoproteins and ATP, GTP, Creatine phosphate, etc. Phosphate
is important for activity of adenylate cyclase, 25-hydroxy vitamin
D-1α-hydroxylase and those involved in 2, 3-diphosphoglycerate.
Hypophosphatemia
This may be caused by (1) shift of
phosphate from extracellular to intracellular space due to increase in glucose,
insulin, respiratory alkalosis, in this condition there is carbohydrate induced
stimulation of insulin secretion, which increases the transport of glucose and
phosphate into insulin-sensitive cells, where it is incorporated into sugar
phosphates and ATP. Respiratory alkalosis cause increase in pH that activates
phosphofructokinase and accelerates glycolysis causing shift of phosphate into
the cell. (2) renal phosphate wasting due to hyperparathyroidism, renal tubular
defects, fanconi syndrome, (3) decreased intestinal absorption as in vomiting,
diarrhea, malabsorption syndrome, vitamin D deficiency. (4) loss from
intracellular phosphate as in ketoacidosis, lactic acidosis.
Hypophosphatemia impair cellular function due to inadequate glycolysis, ATP formation, there is muscle weakness, decreased cardiac output, in RBC there will be decreased 2, 3-DPG which causes tissue hypoxia and severe hypophosphataemia cause hemolysis.
 |
| Fig. Concept Map : Hypophsphatemia |
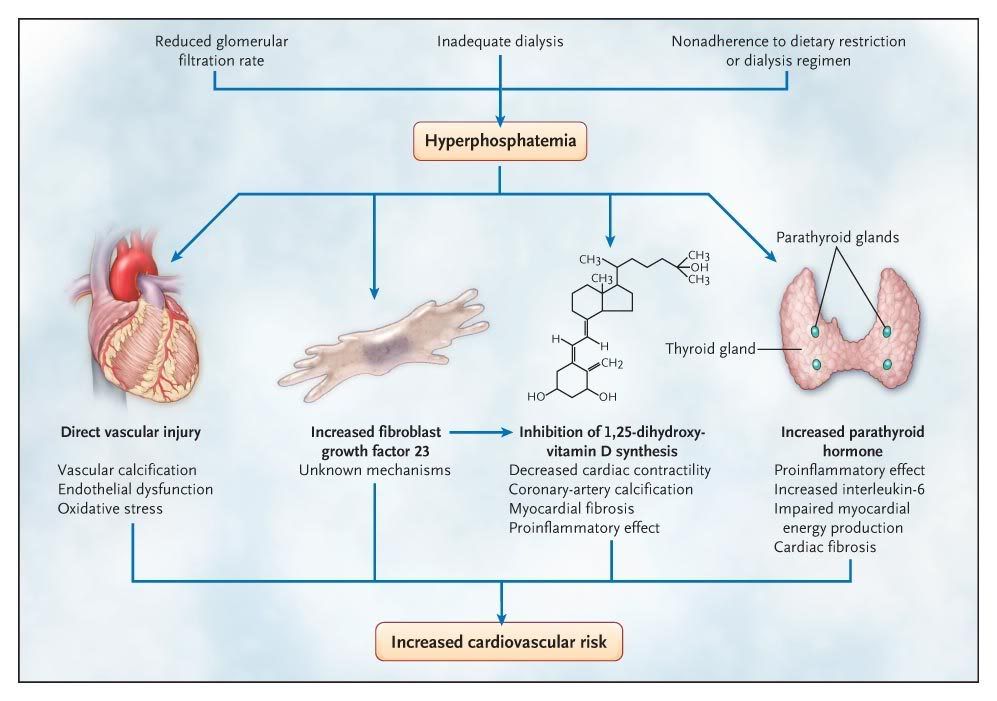
Hyperphosphataemia
Hyperphosphataemia is usually
secondary to the inability of the kidney to excrete phosphate as occurs in
renal failure. Acidosis can also lead to hyperphosphatemia due to hydrolysis of
intracellular organic phosphate containing compounds.
MEASUREMENT
Measurements of inorganic phosphate
are based on the reaction of phosphate ions with ammonium molybdate to form a
phosphomolybdate complex that is measured spectrophotometrically. The
colourless phosphomolybdate complex is measured directly by UV absorption (340
nm), or reduced to molybdenum blue and measured at 600 to 700 nm. An acidic pH
is maintained by addition of acid. Use of sulphuric acid reduces and produces
blue phosphomolybdate complex. Many enzymatic methods are also developed.
Serum or heparinized plasma is
preferred specimens for phosphate measurement. Hemolyzed specimens are
unacceptable as RBC contains high organic phosphate esters which are hydrolyzed
to inorganic phosphate during prolonged storage. Long storage can increase
phosphate concentration. Hemolysis, icterus and lipemia can interfere.
REFERENCE INTERVAL
Serum phosphate in adults = 2.5-4.5
mg/dL (0.81-1.45 mmol/L)



No comments:
Post a Comment