Fatty acyl CoA formed has two fates one, it can undergo β oxidation and the other is it is converted to Tg and phospholipid in the cytosol.
Malonyl CoA (first intermediate in the cytosolic biosynthesis of long-chain fatty acids from acetyl-CoA) inhibit the carnitine acyltransferase I ensures that FA oxidation is inhibited when liver is amply supplied with glucose as fuel and is actively making Tg from excess glucose.
When NADH/[NAD+] ratio is high (energy sufficiency), β-hydroxyacyl-CoA dehydrogenase is inhibited; in addition, high concentrations of acetyl-CoA inhibit thiolase.
During vigorous muscle exercise or during fasting [ATP/AMP] ratio decreases, this activates Protein Kinase (AMPK) which phosphorylates and deactivates acetyl-CoA Carboxylase; this lowers the concentration of malonyl-CoA, relieving the inhibition of fatty acyl-carnitine transport to mitochondria and allow β oxidation to replenish the supply of ATP.
Transcription factors turn on the synthesis of proteins for lipid catabolism
PPAR or PPARα (Peroxisome proliferator-activated receptors) these are transcription factors. During increase demand for energy from fat catabolism, during fast between meals, long starvation. They turn on genes essential for fatty acid oxidation.
In fetus the principle fuels are glucose and lactate, but in the neonatal heart, fatty acids are the main fuel, so during transition from fetus to neonate, its activation, activates the genes essential for fatty acid metabolism.
Endurance training increases PPARα expression in muscle, leading to increase levels of fatty acid oxidizing enzymes.
Glucagon, in low blood glucose, can act through cAMP and the transcription factor CREB to turn on genes for lipid catabolism.
β Oxidation in Peroxisomes
One difference between the Peroxisomal and mitochondrial pathways is in the chemistry of the first step. In peroxisomes, the flavoprotein acyl-CoA oxidase that introduces double bond directly passes electrons to O2 producing H2O2 and this is cleaved by catalase. But in mitochondria electron are transferred to ETC, producing ATP and water. In peroxisomes energy is released as heat. Another difference is mitochondrial NADH is regenerated but in peroxisomes NADH cannot be regenerated. The Peroxisomal system is active on very long chain fatty acids as hexacosanoic acid and on branched chain fatty acids like phytanic acid and pristanic acid. Peroxisomal beta oxidation shortens the side chain of cholesterol in bile acid formation. Peroxisomes also take part in the synthesis of glycerolipids, cholesterol; and dolichol. They do not contain carnitine palmitoyltransferase.
The inability to oxidize these compounds is responsible for several serious human diseases. Individuals with Zellweger syndrome are unable to make peroxisomes.
In mammals, high concentration of fats in the diet results in increased synthesis of enzymes of Peroxisomal beta oxidation in the liver also the hypolipidemic drug like clofibrate. Liver peroxisomes do not contain the enzymes of CAC and cannot catalyze the oxidation of acetyl-CoA to CO2.
α- and ῳ-oxidation
This occurs in some vertebrates and other species. α-oxidation removes the one carbon from carboxyl end and seen in brain tissue. It does not require CoA and does not generate energy. This occurs when beta position is occupied by methyl group e.g. in phytanic acid which cannot undergo beta oxidation.
ῳ-oxidation is brought about by cytochrome P450 and electron donor NADPH in ER of liver and kidney. In mammalian this is minor but when beta oxidation is defective this comes into play. This is also a type of mixed function oxidase reaction. The –CH3 is converted to –CH2OH that is oxidized to –COOH, forming dicarboxylic acid. This is beta oxidized to adipic (C6) and suberic (C8) acids, and excreted in urine.
Ketone body formation
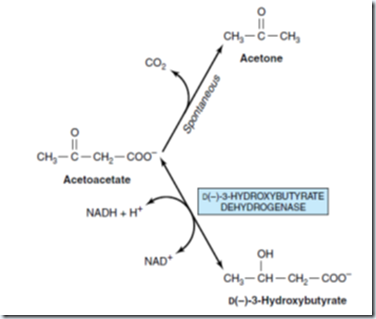
Ketone bodies are acetoacetate, D(-)-3hydroxybutyrate (β-hydroxybutyrate) and acetone (spontaneous decarboxylation of acetoacetate. They are produced in the liver.
|
|
The ratio of 3-hydroxybutyrate/acetoacetate in blood varies between 1:1 and 10:1 in blood.
Formation, utilization, and excretion of ketone bodies. Solid arrow indicates the main pathway.
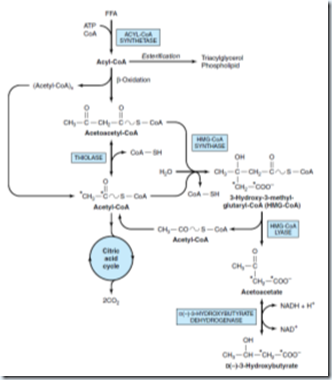
Enzymes for ketogenesis are located in mitochondria. C2 units formed in β-oxidation condense to form acetoacetate. This may occur by inhibition of thiolase. Acetoacetyl-CoA is the starting material for ketogenesis which may arise by β-oxidation or by condensation of 2 acetyl-CoA. 3-hydroxybutyrate is the predominant ketone body present in the blood and urine in ketosis.
Utilization of ketone bodies
In the liver cytosol it is a precursor of cholesterol synthesis. Before utilization, Acetoacetate is activated as Acetoacetyl-CoA involving succinyl-CoA and the enzyme succinyl-CoA-acetoacetate CoA transferase.
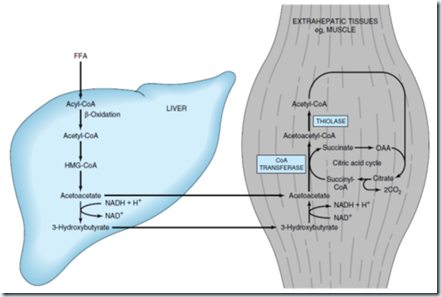
Ketone bodies are oxidized in extrahepatic tissue. If the blood level is raised, oxidation increases until, until a concentration is approximately 12 mmol/L, where they saturate the oxidative machinery. Ketonemia is the increased production of ketone bodies and decreased utilization in extrahepatic tissues.
Regulation of ketogenesis
1. Control in adipose tissue: Free fatty acids produced by lipolysis of TAG in adipose tissue are the precursors of ketone bodies in the liver. The liver both in fed and in fasting condition can extract 30% of FFA passing through it.
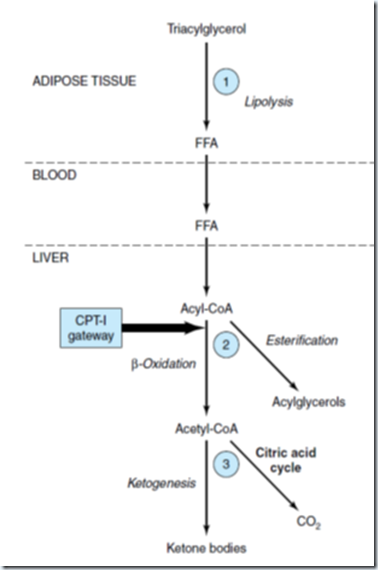
2. Carnitine palmitoyltransferase-I (CPT-1): Regulates the entry of long chain fatty acyl groups into mitochondria prior to β-oxidation. Its activity is low in fed state, leading to depression of fatty acid oxidation, and high in starvation, allowing fatty acid oxidation to increase. During fed state Malonyl-CoA formed inhibits CPT-1, so fatty acids entering liver are esterified as acylglycerols and transported out of liver as VLDL. As the concentration of FFA increases with starvation, acetyl-CoA carboxylase is inhibited by acyl-CoA, and malonyl-CoA decreases, releasing the inhibition of CPT-1 and thus more FFA are oxidized. These events are reinforced in starvation by decrease in the [insulin]/ [glucagon] ratio. This cause inhibition of acetyl-CoA carboxylase in liver by covalent phosphorylation. Thus, β-oxidation is controlled by CPT-1 gateway into the mitochondria, and those not oxidized is esterified.
3. Regulation of partition of acetyl-CoA between the ketogenic pathway and pathway of oxidation to CO2. The partition of acetyl-CoA between the ketogenic pathway and the pathway of oxidation to CO2 is so regulated that the total free energy captured in ATP which results from the oxidation of free fatty acids remains constant. Here the complete oxidation of 1 mol of palmitate involves a net production of 129 mol of ATP via β-oxidation and CO2 production in the citric acid cycle, whereas only 33 mol of ATP are produced when acetoacetate is the end product and only 21 mol when 3-hydroxybutyrate is the end product. Thus, ketogenesis may be regarded as a mechanism that allows the liver to oxidize increasing quantities of fatty acids within the constraints of a tightly coupled system of oxidative phosphorylation without increasing its total energy expenditure. Also increased [NADH]/ [NAD+] ratio caused by β-oxidation caused the decrease in the concentration of oxaloacetate; this can impair the ability of the CAC to metabolize acetyl-CoA.
Regulation of ketogenesis
Regulation of long-chain fatty acid oxidation in the liver
CLINICAL ASPECTS
a) Carnitine deficiency: Particularly in newborn and especially in preterm infants owing to inadequate biosynthesis or renal leakage. There are episodic periods of hypoglycemia owing to reduced gluconeogenesis resulting from impaired fatty acid oxidation in the presence of raised plasma FFA, leading to lipid accumulation with muscular weakness. Treatment is by oral supplementation of carnitine.
b) Inherited carnitine palmitoyltransferase-I deficiency: Affects only the liver, resulting in reduced fatty acid oxidation and ketogenesis with hypoglycemia.
c) Carnitine palmitoyltransferase-II deficiency: Affects skeletal muscles (weakness and necrosis with myoglobinuria) and more severely the liver.
d) Acute fatty liver of pregnancy: Deficiency of long-chain 3-hydroxyacyl-CoA dehydrogenase.
e) Jamaican vomiting sickness: Caused by eating the unripe fruit of the akee tree, which contains a toxin hypoglycin that inactivates medium and short-chain acyl-CoA dehydrogenase.
f) Dicarboxylic aciduria: Excretion of C6-C10 omega dicarboxylic acids and by non-ketotic hypoglycemia. Caused by lack of mitochondrial medium-chain acyl-CoA dehydrogenase.
g) Refsum’s disease: Rare neurological disorder caused by accumulation of phytanic acid, formed from phytol, a constituent of chlorophyll, this occurs due to defect in α-oxidation.
h) Zellweger’s (cerebrohepatorenal) syndrome: There is inherited absence of peroxisomes in all tissues. They accumulate C26 –C38 polyenoic acids in brain tissue, they also have impaired bile acid and ether lipid synthesis.
i) Ketoacidosis: This occurs during starvation, due to depletion of carbohydrate coupled with mobilization of FFA, this process is exaggerated during diabetes mellitus. This also occurs in high fat diet and severe exercise in the postabsorptive state.
Fatty acid contains a long hydrocarbon chain and a terminal carboxylate group. Fatty acids have four major physiological roles.
1. Stored as triacylglycerols (neutral fats) – used as energy reserve
2. FA are building blocks of phospholipids and glycolipids – component of biological membrane.
3. FA covalently attaches protein and modifies them and targets them to membrane locations. E.g. attachment of soluble proteins to membrane by attaching to palmitoyl group, farnesyl group and glycosylphosphatidylinositol group.
4. Fatty acid derivatives serve as hormones and intracellular messengers.
FATTY ACID SYNTHESIS AND DEGRADATION MIRROR EACH OTHER CHEMICALLY BUT DIFFER MECHANISTICALLY.
Triacylglycerols are highly concentrated stores of metabolic energy because they are reduced and anhydrous. The yield from the complete oxidation of fatty acids is about 9 kcal g-1 (38 kJ g-1), in contrast with about 4 kcal g-1 (17 kJ g-1) for carbohydrates and proteins. The basis of this large difference in caloric yield is that fatty acids are much more reduced. Furthermore, triacylglycerols are nonpolar, and so they are stored in a nearly anhydrous form, whereas much more polar proteins and carbohydrates are more highly hydrated. In fact, 1 g of dry glycogen binds about 2 g of water. Consequently, a gram of nearly anhydrous fat stores more than six times as much energy as a gram of hydrated glycogen, which is likely the reason that triacylglycerols rather than glycogen were selected in evolution as the major energy reservoir. Consider a typical 70-kg man, who has fuel reserves of 100,000 kcal (420,000 kJ) in triacylglycerols, 25,000 kcal (100,000 kJ) in protein (mostly in muscle), 600 kcal (2500 kJ) in glycogen, and 40 kcal (170 kJ) in glucose. Triacylglycerols constitute about 11 kg of his total body weight. If this amount of energy were stored in glycogen, his total body weight would be 55 kg greater. The glycogen and glucose stores provide enough energy to sustain biological function for about 24 hours, whereas the triacylglycerol stores allow survival for several weeks.
FFA, monoacylglycerol transported to intestine and absorbed into plasma membrane
In mucosal cells TAG and MAG are resynthesized and packaged into chylomicrons along with apo-B48.
Chylomicrons released into blood via lymph
Action of lipoprotein lipase in adipose tissue and muscle tissue breaks TAG into fatty acids
TAG resynthesized and stored or used as energy
Difference between Fatty acid synthesis and Degradation.
1.Synthesis takes place in the cytosol, in contrast with degradation, which takes place
primarily in the mitochondrial matrix.
2. Intermediates in fatty acid synthesis are covalently linked to the sulfhydryl groups of an
acyl carrier protein (ACP), whereas intermediates in fatty acid breakdown are covalently
attached to the sulfhydryl group of coenzyme A.
3. The enzymes of fatty acid synthesis in higher organisms are joined in a single polypeptide
chain called fatty acid synthase. In contrast, the degradative enzymes do not seem to be
associated.
4. The growing fatty acid chain is elongated by the sequential addition of two-carbon units
derived from acetyl CoA. The activated donor of two carbon units in the elongation step is
malonyl ACP. The elongation reaction is driven by the release of CO2.
5. The reductant in fatty acid synthesis is NADPH, whereas the oxidants in fatty acid
degradation are NAD+ and FAD.
6. Elongation by the fatty acid synthase complex stops on formation of palmitate (C16).
Further elongation and the insertion of double bonds are carried out by other enzyme
systems.






No comments:
Post a Comment